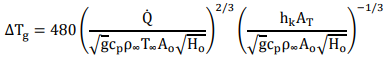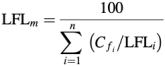Be Prepared For The Toughest Questions
Practice Problems
(Q1)
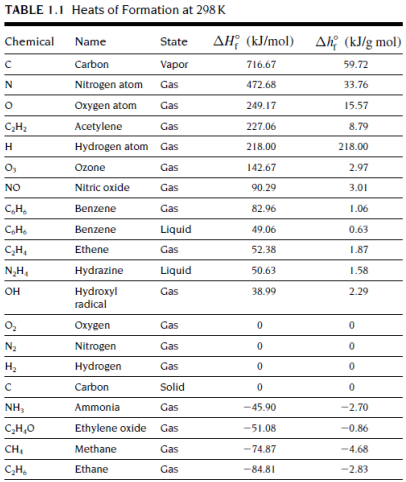
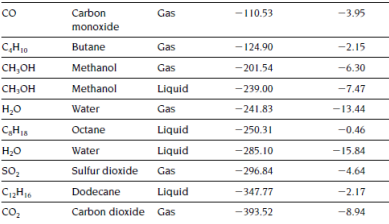
(a) Calculate the heat of combustion (kJ/mol) of ethane (C2H6) in gaseous state for complete combustion at 298K based on the given data on heat of formation in the following table.
(b) Write down the chemical reaction for incomplete combustion of ethane with CO and water vapour as the only products and hence estimate related heat released per mole of methanol consumed at 298K.
(c) Discuss the errors for estimating possible heat released in real fire cases based on the results calculated in part (a) and (b).
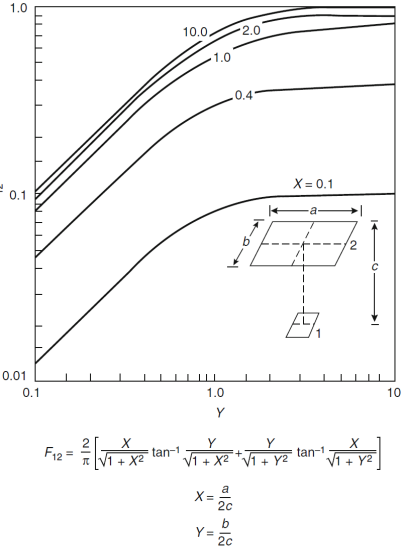
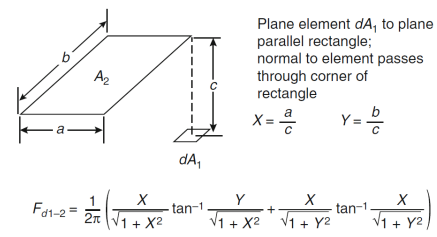
(Q2) Based on View Factor F12 and View Factor Fd1-2., establish the corresponding view factor (in 3 decimal places) between ceiling and a small fire on floor level at different locations (i) at centre, (ii) at the middle of side wall and (iii) at the corner of room with dimensions 1mx1mx1m
Source: Figure A.2 and A.6, SFPE, 2016, SFPE Handbook of Fire Protection Engineering 5th Edition, Appendix A, Pages 3477 and 3481
(Q3) Based on the equation used to calculate upper layer temperature as shown in the following:
where ΔTg is the upper gas temperature rise above ambient, K;
Q is the heat release rate of fire, kW
g is the gravitational acceleration, 9.8 m/s2
cp is the specific heat capacity of air, 1 kJ/(kg K)
ρ is the density of air, 1.18 kg/m3
T is the ambient air temperature, 310 K
Ao is the opening area, m2
Ho is the opening height, m
AT is the total area of compartment enclosing surface, m2
hk is the heat transfer coefficient, 0.03 kW/m2K
Calculate the upper-layer temperature of a room 3m x 3m in floor area and 2.4m high with a door opening 2m high and 1m wide. The fire source is a steady 700kW fire.
Assuming all geometry of the room, fire source and air properties are identical in 280K (i.e. air specific heat capacity of air and density are the same), please estimate the upper gas temperature if the surrounding air is 280K, which is considered a fire occurrence in the winter. Hence, discuss the corresponding impact on fire engineering analysis if the ambient temperature is various in different seasons.
(Q4) Discuss and explain what is flammability limit. Based on the generalisation of Le Chatelier’s rule by Coward et al.
where
Cfi is the volume percent of fuel gas i in the fuel gas mixture.
LFLi is the lower flammable limit concentration of fuel gas i
Estimate the lower flammable limit of a fuel gas with a mixture of 60% methane 20% carbon monoxide and 20% hydrogen mixed with air. (The LFLs of methane, carbon monoxide and hydrogen are 5.0%, 12.5% and 4.0% by volume, respectively)
(Q5) A compartment with the following data is subjected to a fire. The compartment located in remote area is designed as office usage. The following information is given:
Automatic smoke alarm is provided;
No independent water supplies are provided;
No firefighting devices are provided;
The office is made of concrete, ρ=2500kg/m3 ; cp=980J/kgK; k=1.5W/mK;
There are two ventilation openings on the walls. The dimensions (breadth x height) are 3.6m x 1.5m for Opening 1 and 7.2m x 1.5m for Opening 2;
The breadth x length x height of the compartment is 7m x 14m x3m;
Use Annex E of the Eurocode 1(Part 1-2) to determine the fire load density (MJ/m2 ) incorporating appropriate factor of safety.
Plot the temperature-time curve of the compartment fire for this office.
(Q6) Please list and explain the tenability criteria that is commonly used in the computational fluid dynamic (CFD) models by fire engineering design approach for the ASET study in accordance with the Hong Kong FS Code (Code of Practice for Fire Safety in Building 2011). Please also try to explain the impacts of the fire on the human body on the basis of the criteria you listed above.
(Q7) Calculate the heat energy releases just before reaching the maximum heat release rate, and during the steady burning state for 50kg of rubber tyre with calorific value of 32MJ/kg. Assuming the maximum heat release rate is 10MW and the growth constant k=150s/√MW. What would happen if the growth constant k changes to 600s/√MW?
(Q8) Discuss and compare the ‘standard temperature-time curve (ISO834)’ and the ‘parametric temperature-time curve (based on EN 1991-1-2)’. Assume that you are a fire engineer for a design task of structural fire resistant design in several compartment fire scenarios. How do you plan to incorporate both aforementioned fire curves in your work?
(Q9) (a) List three types of fire engineering design tools for fire and smoke simulation models which are commonly adopted in the performance based fire engineering approach in Hong Kong
(b) Briefly explain their features and difference in use
(c) In your opinion, which fire and smoke simulation model you mentioned above would be adopted in the fire engineering design of an atrium in a shopping center having irregular shape and the 35 m ceiling height? Briefly explain why you choose this model for this type of building structure.
Know the process
Students succeed in their courses by connecting and communicating with
an expert until they receive help on their questions

Unable to find what you’re looking for?
Consult our trusted tutors.


 Login | Sign Up
Login | Sign Up